Gas Laws Worksheet #1
Look at the axis on each graph and tell me the independent variable, the dependent variable, and R = 0.08206 l atm / mol k
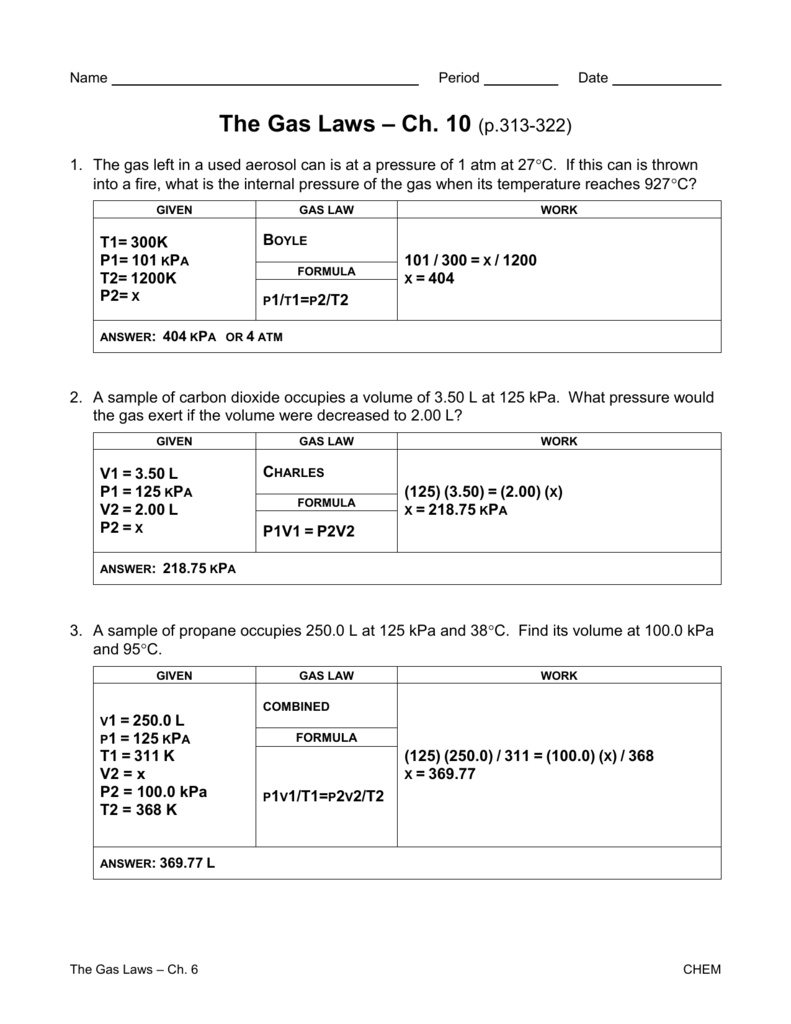
Gas Laws Worksheet 1 Answer Key —
The reader is tasked with filling from the blanks inside a published piece or sentence.
Gas laws worksheet #1. Free tales followed by workouts, in addition to worksheets on particular comprehension subjects. What will its volume be at 20.0 °c and 780.0 mm of mercury pressure? What will the volume of the.
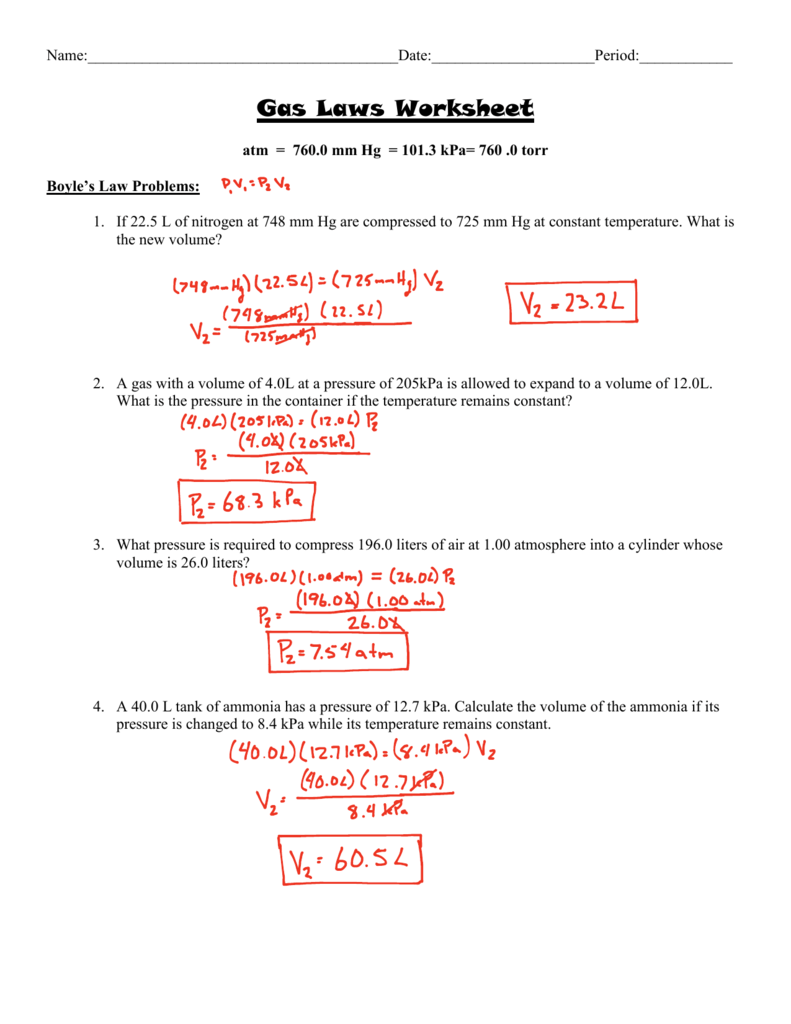
The simulation (15 minutes) we are going to study 2 of the famous gas laws: A gas balloon has a volume of 106.0 liters when the temperature is 45.0 °c and the pressure is 740.0 mm of mercury. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature.
Each of the chemical equations must first be balanced. The gases in a hair spray can are at a temperature of. Combined gas law worksheet 1 answers.
Gas laws ideal gas law homework ideal gas law chemistry classroom science chemistry. 2) a toy balloon has an internal pressure of 1.05 atm and a volume of 5.0 l. Use the gas laws we have learned to solve each of the following problems.
Formulas & masses (worksheets) solutions. Gas stoichiometry worksheet 1 name: If the temperature where the balloon is released is 20 °.
A 1.25 mole sample of a gas occupies a volume of 28.9 l. Use our studying comprehension worksheets to improve studying comprehension. The value of r varies with the units chosen:
By amanda on february 20, 2022. Combined gas law worksheet 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4 atm? 6) explain why an unopened bag of chips left in a.
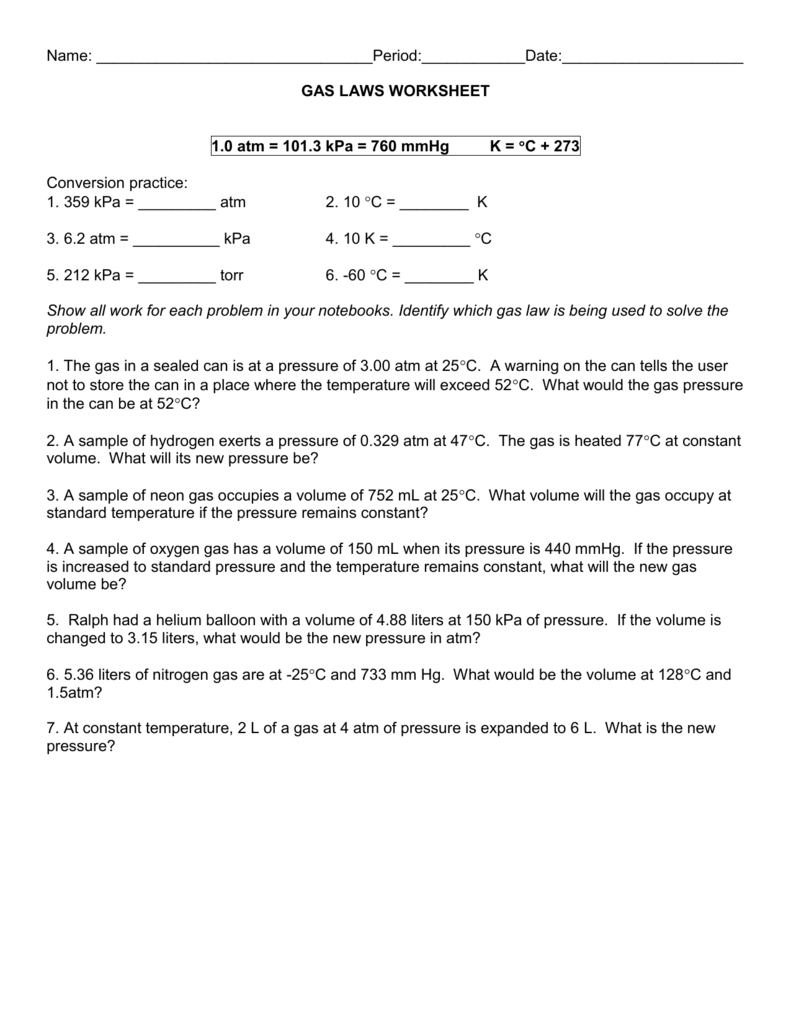
1 atm = 760.0 mm hg = 101.3 kpa k = 273 +oc. Gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr. 2) describe charles' law (what's constant, trend,.
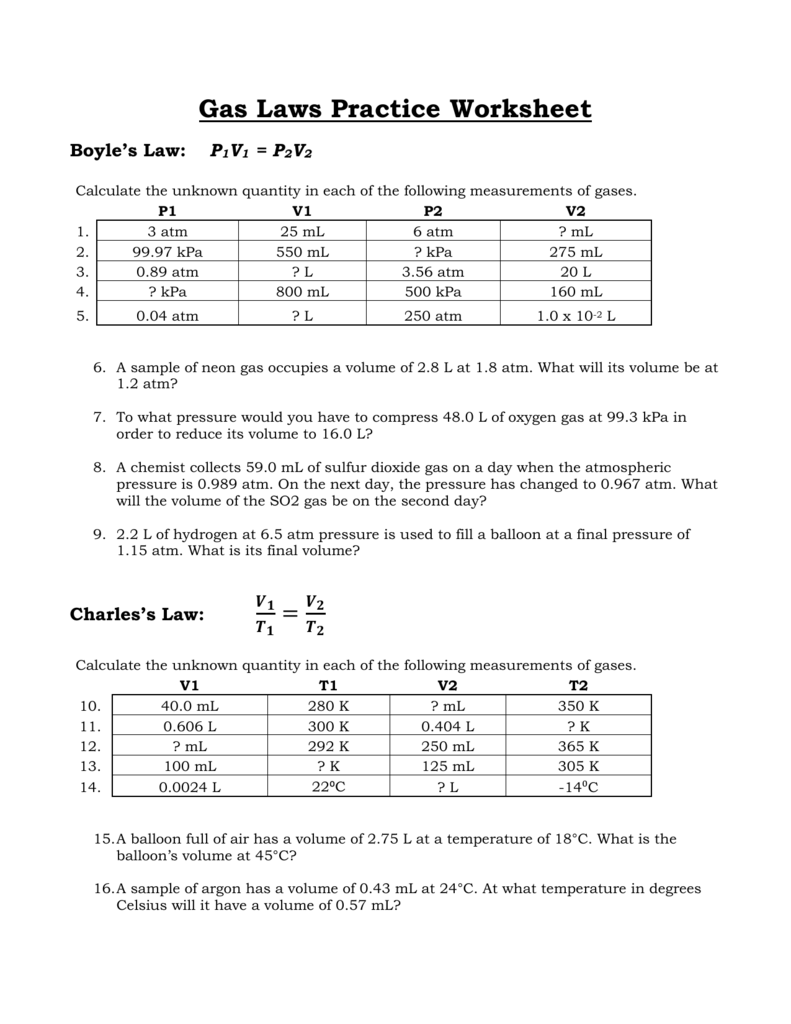
A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. What is the volume of hydrogen at a pressure of 1.06 atm if 200 cm3 of hydrogen is collected at a pressure of 1.00 atm? Gas laws practice worksheet charles' law 1) if i have 45 liters of helium in a balloon at 25°c and increase the temperature of the balloon to 55°c, what will the new volume of the balloon be?
1) describe boyle's law (what's constant, trend, relationship?). If 400 cm3 of oxygen is collected at a pressure of 980 mmhg, what volume will the gas occupy if the pressure were changed to 940 mmhg? Kmol if pressure is needed in kpa then convert by multiplying by 1013kpa 1atm to get r 831 lkpa kmole 1 if i have 4 moles of a gas.
Use the gas laws to explain whether the effect will be greater when the liquid is warm or cold? When calcium carbonate is heated strongly, carbon dioxide gas is released according to the. Show all your work for credit.
Pv = nrt p = pressure v = volume n= moles of gas, r = universal gas constant t = temperature. What is the new volume? Determine the total pressure of a gas mixture that contains oxygen at a pressure of 150.mmhg, nitrogen at 350.mmhg pressure, and helium at a pressure of 200.mmhg.
Using si units of kilograms, meters, and seconds with these fundamental equations, determine the combination of units that define the following: Simulation worksheet 2 screen 3: A helium balloon is filled to a volume pf 5.60 l at 25 °c.
Ch 14 ideal gas law kinetic theory 16 638 jpg 638 479 ideal gas law high school chemistry chemistry class. Boyle's law, which looks at the relationship between pressure and volume, and charles's law, which looks at the relationship between volume and temperature. This means that all units must agree with this gas constant.
Download the ios download the android app chem 120 gas laws practice questions gas constant r = 0.0821 l atm/ mol k 1. N = pv = (2.8 atm)(98 l) = 11 moles of gas rt (0.0821 l.atm/mol.k)(292 k) 2) if 5.0 moles of o 2 and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 0 2) calcium carbonate decomposes at 1200°c to form carbon dioxide and calcium oxide.
If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. The pressure on 2.50 l on anesthetic gas changes from 760 mmhg to 304 mmhg. Gas law worksheet #2 (dalton's law and ideal gas law) dalton's law:
A gas mixture containing oxygen, nitrogen, and carbon dioxide has a. Gas laws worksheet atm = 760.0 mm hg = 101.3 kpa= 760.0 torr boyle's law problems: February 20, 2022 on ideal gas law practice problems worksheet answers.
View gas laws lab report sheet(1) (autorecovered).docx from nurs 101 at university of louisville. Each of these laws can be derived from this law. Gas laws 1 (worksheet) last updated.
P t = p 1 + p 2 + p 3 +.
8 Best Images of Chemistry Gas Laws Worksheet Ideal Gas
32 Mixed Gas Law Worksheet Answers Free Worksheet
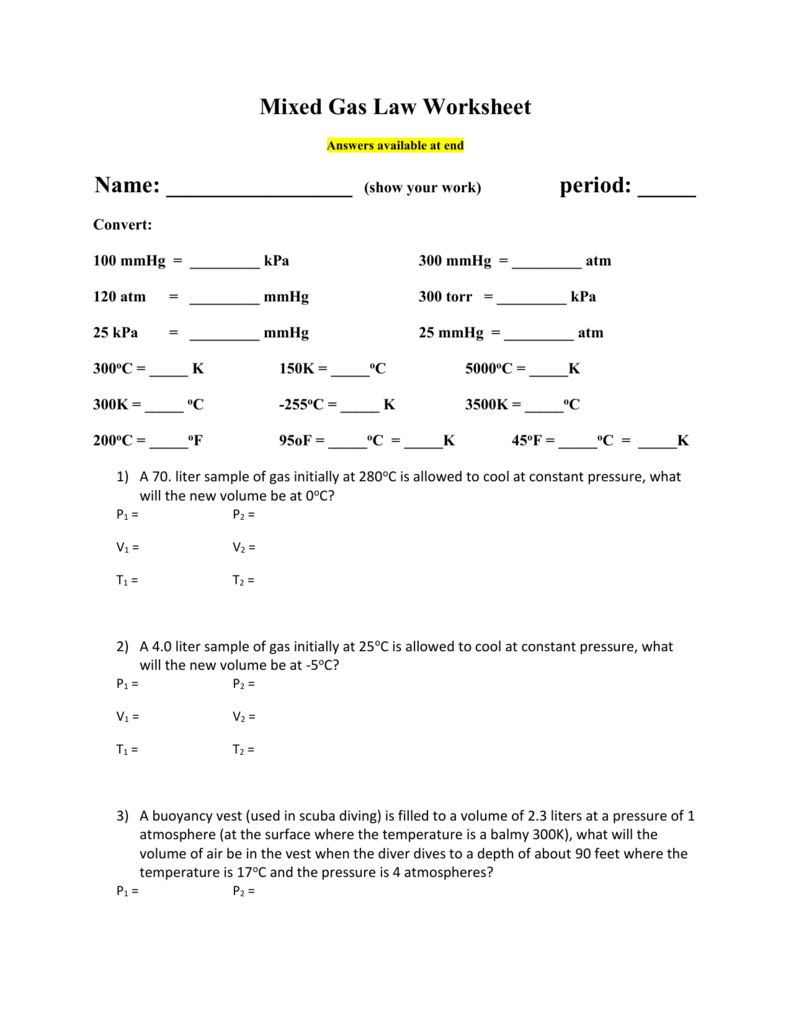
Mixed Gas Law Worksheet Answers available at end Name (show

Gas Laws Worksheet 1 Answer Key

Gas Laws And Scuba Diving Worksheet Answers /

The Combined Gas Law Worksheet for 10th Higher Ed

Gas Laws Supplemental Worksheet
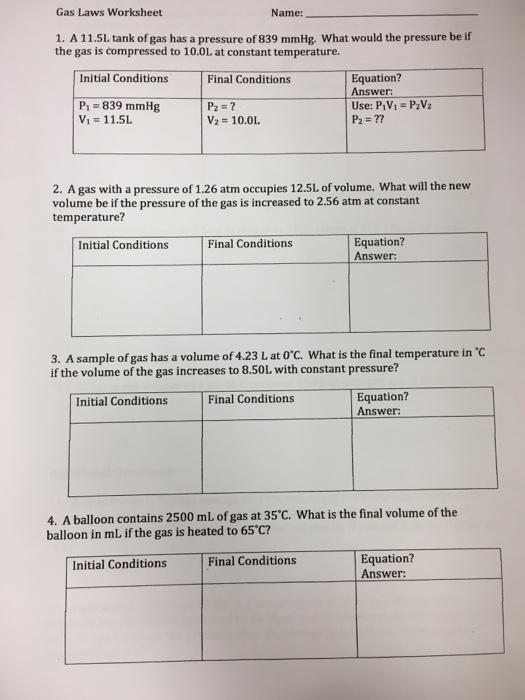
Solved Gas Laws Worksheet Name 1. A 11.5L Tank Of Gas Ha
Gas Laws Worksheet III Answer Key 1112 Gases Mole (Unit)

Mixed Gas Laws Worksheet Answers
worksheet. Combined Gas Law Worksheet Answers. Worksheet
33 Mixed Gas Law Worksheet Answers Worksheet Resource Plans

Gas laws worksheet 1 answer key,

26 Ideal Gas Law Worksheet Answer Key Worksheet Information
Worksheet_2(Gas Laws, Density, Molar Mass)(1)(1) Mole